Abstract
Background: Guideline recommendations from the Infectious Diseases Society of America (IDSA) and the 4th European Conference on Infections in Leukemia (ECIL-4) for the optimal duration of empiric antimicrobial therapy in patients with hematological malignancies and febrile neutropenia (FN) of unknown origin (FUO) vary given limited available evidence. Recent studies involving hematology patients and hematopoietic cell transplantation (HCT) recipients with FN have demonstrated that de-escalation of broad-spectrum antimicrobials (BSA) prior to hematopoietic recovery is associated with greater antibiotic-free days, but without increased risks, such as recurrent fever, bacteremia, intensive care unit (ICU) admission, or inpatient mortality. However, the safety of this de-escalation approach has not been extensively studied in autologous and haploidentical HCT recipients within the United States as most prior studies were conducted in Europe. The main purpose of this study was to compare rates of recurrent fever, re-escalation of therapy, and Clostridium difficile-associated infection (CDI) in autologous and allogeneic HCT recipients with FUO who received early de-escalation of BSA prior to hematopoietic recovery versus those who continued BSA until hematopoietic recovery.
Methods: This retrospective, observational study assessed HCT recipients with FN admitted to Carolinas Medical Center in Charlotte, North Carolina between March 2014 to April 2018. Patients were included if they were ≥ 18 years of age, had an active hematologic malignancy and underwent allogeneic or autologous HCT, experienced their first episode of FN after HCT, and were initiated on appropriate BSA for ≥ 48 hours. Patients were excluded if they had microbiological or radiological diagnosis of active bacterial, fungal, or viral infection during the FN episode. Patients were enrolled into either cohort 1, which represented patients who were de-escalated to prophylactic antimicrobials prior to hematopoietic recovery (early de-escalation group), or cohort 2, which represented patients who continued BSA until hematopoietic recovery (hematopoietic recovery de-escalation group). Fisher's exact test was conducted to make cohort comparisons for categorical patient characteristics, while Mann-Whitney U test was employed for continuous variables. Multivariate logistic regression was utilized to evaluate rates of recurrent fever, re-escalation of therapy, and CDI between the 2 cohorts.
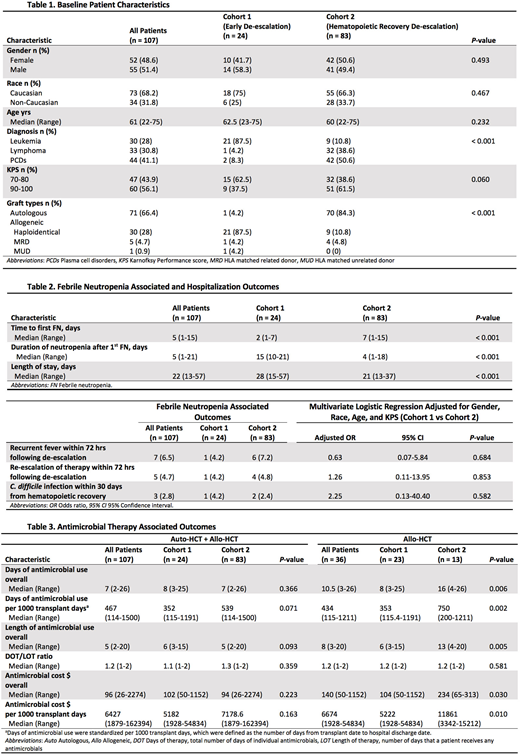
Results: A total of 107 patients were included with 24 (22.4%) in cohort 1 and 83 (77.6%) in cohort 2. Most patients (87.5%) in cohort 1 received haploidentical HCT, whereas 84.3% of patients in cohort 2 received autologous HCT (P < 0.001). The median duration of neutropenia following the first FN episode was significantly shorter in cohort 2 (15 vs 4 days in cohorts 1 and 2, respectively, P < 0.001). There were no significant differences in rates of recurrent fever (4.2% vs 7.2%, adjusted OR (AOR) = 0.63,P = 0.684) and re-escalation of therapy (4.2% vs 4.8%, AOR = 1.26, P = 0.853) within 72 hours following de-escalation between the 2 cohorts. Rates of CDI within 30 days from hematopoietic recovery were also similar (4.2% vs 2.4%, AOR = 2.25, P = 0.582). No patients experienced inpatient mortality, ICU admission, or bacteremia. There were no significant differences in days of antimicrobial use per 1000 transplant days (P = 0.071).
In a subgroup analysis of only allogeneic HCT recipients, haploidentical was the most common transplant type with 91.3% (n = 21/23) in cohort 1 and 69.2% (n = 9/13) in cohort 2. Recurrent fever was very infrequent among allogeneic HCT recipients (n = 1 in cohort 1 vs n = 0 in cohort 2, P > 0.999). However, a significant reduction in days of antimicrobial use per 1000 transplant days (P = 0.002) was observed in cohort 1 compared to cohort 2.
Conclusion: HCT recipients with FUO who received de-escalation of BSA prior to hematopoietic recovery did not experience increased rates of recurrent fever, re-escalation of therapy, CDI, bacteremia, ICU admission, or inpatient mortality. These results concur with recently published studies and the ECIL-4 guidelines. An early de-escalation approach in haploidentical HCT recipients specifically appears to be safe and may result in a reduction in antimicrobial utilization.
Grunwald:Forma Therapeutics: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ariad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cardinal Health: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Medtronic: Equity Ownership; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees. Avalos:Juno: Membership on an entity's Board of Directors or advisory committees. Usmani:Amgen, BMS, Celgene, Janssen, Merck, Pharmacyclics,Sanofi, Seattle Genetics, Takeda: Research Funding; Abbvie, Amgen, Celgene, Genmab, Merck, MundiPharma, Janssen, Seattle Genetics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal